M. Sc. Maximilian Zäh
Thermodynamic Aspects in the Solubilization of Biopharmaceutics
When it comes to long-term stability of biopharmaceuticals, liquid dosage/storage forms occasionally reach their limits. Freeze-drying / Lyophilization is used to reduce the water content in the formulation. Within this project, we perform a thermodynamic characterization of the freeze-drying process and the investigate influence of additives on the stability and solubilization of the biopharmaceutical.
Description
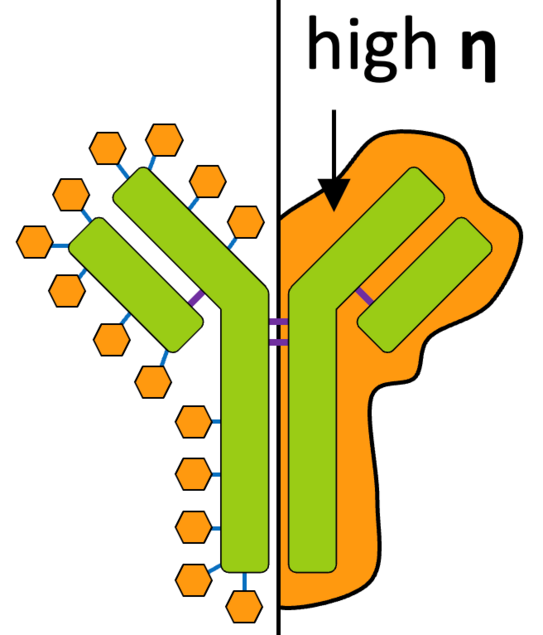
Two different stabilisation theories are discussed for the stabilisation of biopharmaceuticals. A kinetic and an equilibrium-based approach can be assumed. In the kinetic approach, the degradation of the biopharmaceuticals is related to the viscosity of the freeze-dried formulation. In the equilibrium-based approach, the interactions between biopharmaceuticals and additives are identified as decisive factors for solubilization and increased stability. [2]
Both approaches will be investigated in this project and the knowledge gained will be used to accelerate and improve the development of new formulations.
References
| [1] | A. Arsiccio, R. Pisano: “The Preservation of Lyophilized Human Growth Hormone Activity: how Do Buffers and Sugars Interact?” Pharmaceutical research, 2018, 35, 131. |
| [2] | Chang, L.L.; Shepherd, D.; Sun, J.; Ouellette, D.; Grant, K.L.; Tang, X.C.; Pikal, M.J.l: “Mechanism of protein stabilization by sugars during freeze-drying and storage: native structure preservation, specific interaction, and/or immobilization in a glassy matrix? ” Journal of Pharmaceutical Sciences, 2005, 94, 1427–1444. |