Dissolution of Formulated Poorly Water-soluble Pharmaceutical
The development of potentially efficacious pharmaceuticals is hindered by their poor aqueous solubilities which result in a poor bioavailability. Our research focus on the dissolution mechanism and rate of formulated poorly water-soluble pharmaceuticals in solutions with experiments and theoretical modeling. It is expected that the research could provide useful knowledge for the formulation strategy development of poorly water-soluble amorphous pharmaceuticals
Description
It is known that the development of potentially efficacious pharmaceuticals is hindered by their poor aqueous solubilities which result in a poor bioavailability [1]. Amorphous pharmaceuticals hold potential to enhance the dissolution rate for poorly water-soluble pharmaceutical candidates [2]. In recent years, several investigations on the amorphous forms of active pharmaceutical ingredients (APIs) formulated in various polymer carriers to improve their dissolution have been reported. Characterizing the pharmaceutical-dissolution mechanism to measure product performance is particularly important for rationally designing optimal formulations of poorly soluble compounds [3].
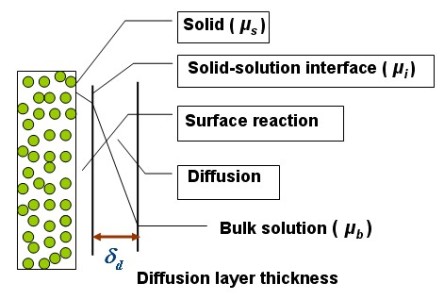
The pharmaceutical dissolution kinetics of formulated poorly water-soluble amorphous pharmaceuticals has been widely investigated. However, it is difficult to obtain the dissolution rate-controlling step just from the experimental dissolution kinetics curve and empirical model of the dissolution. Therefore, in this research, the dissolution kinetics of the poorly water-soluble pharmaceutical is analyzed with both experimental measurements and modeling in which the mass transport of the dissolved pharmaceutical at the solid-solution interface is described.
To achieve this objective, the following investigations are performed:
(a) to prepare the amorphous solid dispersions of pharmaceutical;
(b) to measure the solubility of pharmaceutical in aqueous systems;
(c) to determine the dissolution kinetics of the pharmaceutical experimentally;
(d) to investigate the dissolution mechanism with theoretical modeling in which the Perturbed-Chain Statistical Associating Fluid Theory (PC-SAFT)
Equation of State [4] will be used to represent the thermodynamic properties of the system. It is expected that the research could provide useful knowledge for the formulation strategy development of poorly water-soluble amorphous pharmaceuticals.
References
| [1] | M. Kennedy, J. Hu, P. Gao, L. Li, A. Ali-Reynolds, B. Chal, V. Gupta, C. Ma, N. Mahajan, A. Akrami, and S. Surapaneni: "Enhanced Bioavaolability of a Poorly Soluble VR1 Antagonist Using an Amorphous Solid Dispersion Approach: A Case Study" Molecular Pharmaceutics, vol. 5, pp. 981-993, 2008 |
| [2] | D. Zhou, G. G. Z. Zhang, D. Law, D. J. W. Grant, and E. A. Schmitt: "Thermodynamics, Molecular Mobility and Crystallization Kinetics of Amorphous Griseofulvin" Molecular Pharmaceutics, vol. 5, pp. 927-936, 2008 |
| [3] | C. K. Brown, H. P. Chokshi, B. Nickerson, R. A. Reed, B. R. Rohrs, and P. A. Shah: "Acceptable Analytical Practices for Dissolution Testing of Poorly Soluble Compounds" Pharmaceutical Technology, December, pp. 56-65, 2004 |
| [4] | M. Kleiner, F. Tumakaka, and G. Sadowski: "Thermodynamic Modeling of Complex Systems" Structure and Bonding, Springer-Verlag Berlin Heidelberg, vol. 131, pp. 75-104, 2009 |