Solvate and Hydrate Formation of Pharamaceuticals
Many Active Pharmaceutical Ingredients (API) might be present in different solid states (different polymorphs, hydrates, solvates, salts, or cocrystals) [1]. All those solid forms result in completely different physico-chemical properties and therefore need to be characterized in detail during the development of a pharmaceutical formulation. Commonly, the different solid forms of an API are determined via time-consuming and expensive screening approaches.
Description
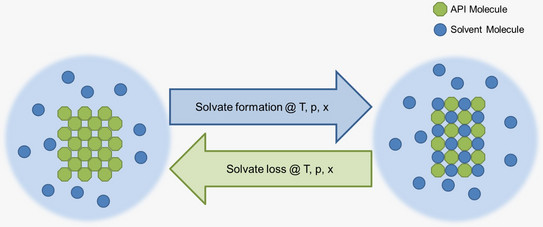
The aim of this work is to investigate the solvate formation of APIs. Solvate formation and stability will be experimentally investigated and thermodynamically modelled with the equation of state Perturbed-Chain Statistical Associating Fluid Theory (PC-SAFT) [2] in order to gain a fundamental understanding of the solvate formation of new APIs.
References
| [1] | W. Jones, W. S. Motherwell and A. V. Trask: "Pharmaceutical Cocrystals" MRS Bulletin, vol. 31(11), pp. 875-879, 2006 |
| [2] | J. Gross and G. Sadowski: "Perturbed-Chain SAFT: An equation of state based on a perturbation theory for chain molecules" Industrial & Engineering Chemistry Research, vol. 40(4), pp. 1244-1260, 2001 |