Pressure and Solvent Influence on Thermodynamic Equilibria and Kinetics of Chemical CO2 Conversions
The development of innovative chemical processes for CO2 conversion into useful platform chemicals, which are in return used as raw materials, has become significantly important for the industry. Since recent methods suffer from the formation of environmentally harmful salt as a waste product, a new salt-waste free method with a coupled C-H carboxylation, subsequent esterification and recycling of the inorganic base overcomes the problems of the unsustainable standard process.[1,2] The aim of this work is to investigate the influence of different solvents, pressure and concentration of the reaction agents on the phase and reaction equilibria, as well as the kinetics of the coupled C-H carboxylation and esterification reactions.
Description
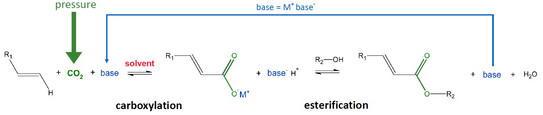
The use of CO2 as a C1 building block for the production of platform chemicals might be one of the most interesting topics in the chemical industry. CO2, as one raw material of the reaction, is available abundantly in the atmosphere and furthermore a side product in many combustion reactions. In order to achieve a sustainable and waste-free process, the inorganic base is recycled by coupling the carboxylation and esterification in a one-pot system. High pressure is applied to reach higher solubility of CO2 in the liquid phase of the reaction system [1]. The overall reaction scheme can be described as follows:
The main goal is to predict the influence of solvent and of pressure on the coupled reactions with the model-supported optimization of the solvent, pressure, temperature, type of base and water content in order to maximize the yield and conversion of the CO2. For that purpose, ePC-SAFT is used to model the required phase equilibria, CO2 and base solubility in the organic solvent, as well as the reaction kinetics of both reactions [3]. This will require a correct treatment of the electrolyte base in organic solvents.
References
| [1] | T. Wendling, E. Risto, T. Krause, L. J. Gooßen: "Salt-Free Strategy for the Insertion of CO2 into C-H Bonds: Catalytic Hydroxymethylation of Alkynes" Chem. Eur. J. 2018, 24, 6019-6024 |
| [2] | J. Hong, M. Li, J. Zhang, B. Sun, F. Mo: "C-H Bond Carboxylation with Carbon Dioxide" ChemSusChem 2019, 12, 6-39 |
| [3] | C. Held, T. Reschke, S. Mohammad, A. Luza, G. Sadowski: "ePC-SAFT revised" Chemical Engineering Research and Design, vol. 92, pp. 2884-2897, 2014 |