Solvent Impact on the Product Quality of Pharmaceutical Formulations
- Research Highlights
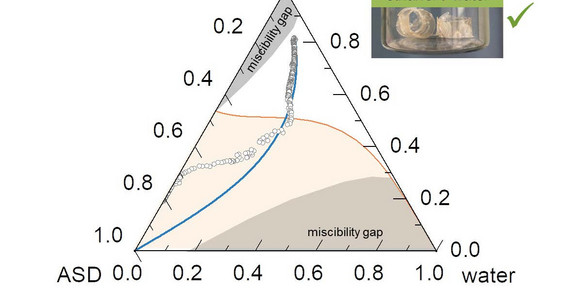
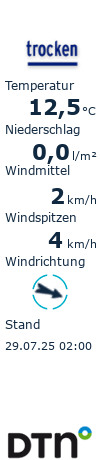
drying. In order to obtain homogeneous and stable formulations, any changes, like crystallization of the active pharmaceutical
ingredient (API) or phase separation of the components involved must be avoided. The phase behavior during drying must
be known for successfully drying the formulation from a solvent or solvent mixture. The proposed method for the first time
enables identifying appropriate solvent candidates for the production of ASDs with significantly less experimental effort than
before.
- ScientificHighlights_2019_SD.pdf PDF (1 MB)