Counteracting the loss of release for indomethacin-copovidone ASDs.
- Research Highlights
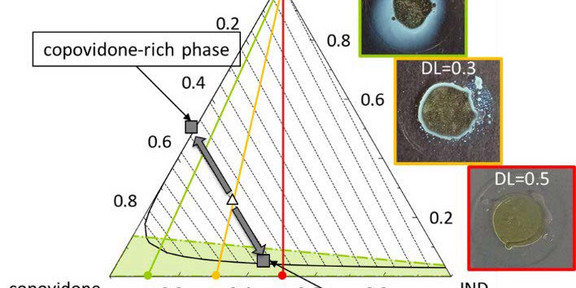
The bioavailability of an active pharmaceutical ingredient (API) with low water solubility can be enhanced by dissolving the API in a polymer matrix generating an amorphous solid dispersion (ASD), which used as tablets. Upon ASD dissolution, the polymer and the API are supposed to simultaneously release from the ASD. However, ASDs often show simultaneous and fast release at low API to polymer ratios (low drug loads (DL)), while ASDs with high DL show a loss of API release. This study explained this phenomenon via investigating the release kinetics and phase behavior of an ASD consisting of the API indomethacin (IND) and the polymer copovidone both experimentally and theoretically. Modeling the experimental release kinetics, we were able to predict the formation of an ASD layer at the ASD-water interface, which almost exclusively contains amorphous indomethacin (IND). Our phase-diagram predictions and experimental data verify that water-induced phase separation during ASD dissolution. Whereas the evolving copovidone-rich phase dissolves, the IND-rich phase remains undissolved and forms a super-hydrophobic layer that covers the remaining inner core of the ASD, thus finally completely preventing its dissolution.
- ScientificHighlights_2024_DB.pdf PDF (382 KB)