Simultaneous Prediction of Co-Solvent Influences on Michaelis Constants and Reaction Equilibrium of Ketone Reductions
- Research Highlights
Biotechnologists commonly apply co-solvents in order to improve enzyme-catalyzed reaction systems. The effect of such
co-solvents on reaction kinetics and reaction equilibrium of enzyme-catalyzed reactions is mainly studied experimentally.
However, this does neither allow explaining nor predicting the observed co-solvent effects on reaction kinetics or equilibrium
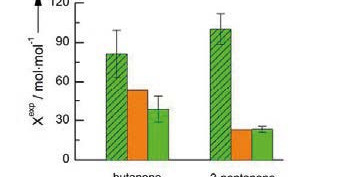
compositions. In this work, the reaction equilibrium and the Michaelis constants of ketone reductions were predicted
with ePC-SAFT. ePC-SAFT showed that adding 17 wt.-% of PEG 6000 is beneficial for reaction kinetics while shifting the
reaction equilibrium backwards to the reactant side. Experimental validation showed that these predictions were in a very
good agreement to the experimental data. As co-solvent – enzyme interactions were not considered for the predictions
co-solvent – reacting agent interactions are decisive for the co-solvent influence on reaction equilibrium and the Michaelis
constants for the considered reactions.