Predicting Kinetics of the PET Glycolysis Reaction using an electrolyte thermodynamics-based framework.
- Research Highlights
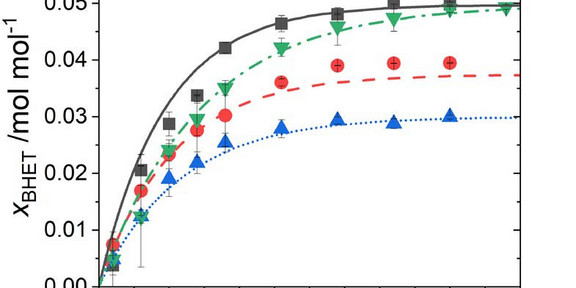
The optimization of the reaction conditions of a chemical reaction usually requires extensive experimental efforts to maximize kinetics. This is caused by the fact that reaction kinetics typically depend on reactant concentration(s), catalyst concentration, temperature, and co-solvents that might be present in the reaction mixture. One of the most important depolymerization reactions is the polyethyleneterephthalate (PET) glycolysis reaction. Currently, only empirical methods are available for modeling PET glycolysis kinetics. Such models do not have any predictive power and thus, these rely heavily on experimental data and are limited to defined operation conditions. In this work, a predictive model was developed aiming at predicting the impact of reactant ratio, catalyst concentration, and co-solvent effects on the equilibrium + kinetics of the PET glycolysis. To this end, the electrolyte equation of state ePC-SAFT was used in an activity-based kinetic framework. The thermodynamic activity of the catalyst zinc acetate was included, enabling the incorporation of catalyst interactions with the liquid environment in the reaction phase. The results showed very promising results, meaning that thermodynamics is highly useful to screen the most promising reaction conditions that allow fast reaction kinetics and maintain the high equilibrium yield of PET glycolysis.