Highly efficient lithium extraction from magnesium-rich brines with ionic liquid-based collaborative extractants
- Research Highlights
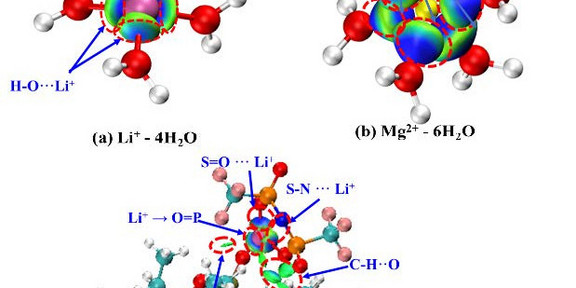
Selective extraction of Li+ from high Mg2+/Li+ ratio brines with ionic liquid (IL) based collaborative extractants was investigated by experiments, thermodynamic analyses, and quantum chemical (QC) calculations. The results demonstrated that the system 1-(2-hydroxyethyl)-3-methylimidazolium bis(trifluoromethylsulfonyl)imide + trioctyl phosphate ([HOEMIM][Tf2N] + TOP) was considered as the best extractant, with the very high extraction efficiency of Li+ (ELi+≈83%) and separation selectivity of Li+/Mg2+ (≈742), which is higher than any known report from the open literature. The thermodynamic model ePC-SAFT was extended to quantitatively predict the phase equilibria of the so-called “organic-inorganic complex strong electrolyte system” presented in this work as well as the related extraction indicator ELi+. The molecular-level extraction mechanism was explored by QC calculation, indicating that the strong multi-site intermolecular interactions between Li+ and [HOEMIM][Tf2N] + TOP break the Li+ hydration.